 |
| There are special properties of light that we can take advantage of to understand even objects that are millions and billions of light years away. In this section we explore some of these properties and how we can use them to understand our Universe. In the previous section of this unit, you were told that superheated material created by the supernova explosion gives off X-rays and gamma-rays. X-rays and gamma-rays are really just light (electromagnetic radiation) that has very high energy. |
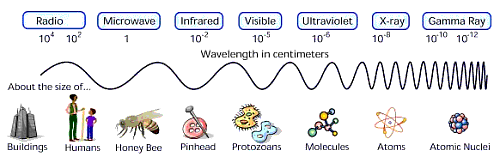
The entire range of energies of light, including both light we can see and light we cannot see, is called the electromagnetic spectrum. It includes, from highest energy to lowest: gamma-rays, X-rays, ultraviolet, optical, infrared, microwaves, and radio waves.
 |
Because light is something that is given off, or radiated from an object, we can call it radiation. That's why we often talk about X-ray radiation - it's the same thing as saying X-ray light. When we refer to the whole spectrum of light, we can call it electromagnetic radiation.
Because we can see only visible light, we are put at a disadvantage because the Universe is actively emitting light at all different energies.
Light has different colors because it has different energies. This is true whether we are talking about red and blue visible light, or infrared (IR) and X-ray light. Of all the colors in the visible spectrum, red light is the least energetic and blue is the most. Beyond the red end of the visible part of the spectrum lie infrared and radio light, both of which have lower energy than visible light. Above the blue end of the visible spectrum lies the higher energies of ultraviolet light, X-rays, and finally, gamma-rays.
Because of light's unique properties, it can be described not only in terms of its energy, but also its wavelength, or its frequency. There is a one-to-one correspondence between each of these representations. X-rays and gamma-rays are usually described in terms of energy, optical and infrared light in terms of wavelength, and radio in terms of frequency. This is a scientific convention that allows the use of the units that are the most convenient for describing whatever energy of light you are looking at. For example, it would be inconvenient to describe both low energy radio waves and high energy gamma-rays with the same units because the difference in their energies is so great. A radio wave can have an energy on the order of 4 x 10-10 eV as compared to 4 x 109 eV for gamma-rays. That's an energy difference of 1019, or ten million trillion eV!
Wavelength is the distance between two peaks of a wave, and it can be measured with a base unit of meters (m) (such as centimeters, or Angstroms). Frequency is the number of cycles of a wave to pass some point in a second. The basic unit of frequency is cycles per second, or Hertz (Hz). Energy in astronomy is often measured in electron volts, or eV or its multiples (such as kilo electron volts, or 1,000 eV) .
Wavelength and frequency are related by the speed of light (c), a fundamental constant. Energy is also directly proportional to frequency (the constant of proportionality is Planck's constant, h) and inversely proportional to wavelength. It was Max Planck who demonstrated that light sometimes behaves as a particle by showing that its energy (E), divided by its frequency (the Greek letter nu) is a constant. Since we know that frequency is equal to the speed of light (c) divided by wavelength (the Greek letter lambda), we also know the relationship between energy and wavelength. The energy (or wavelength or frequency) of light can give important clues into how the light was produced, and it is the characterization of light emission that allows us to understand objects in the distant universe.
Since light can act like both a particle and a wave, we say that light has a particle-wave duality. We call particles of light photons. Low-energy photons (i.e. radio) tend to behave more like waves, while higher energy photons (i.e. X-rays) behave more like particles. This is an important difference because it affects the way we build instruments to measure light (telescopes!).
You are familiar with light in many forms, like sunlight, which you see every day. But how is this light created? And how can we use the properties of light to understand objects in the Universe?
Because astronomical objects emit light at different energies of light, it pays to make multiple observations of these objects. Supernovae remnants can give off visible light, ultraviolet light, radio waves and X-rays. And each observation of a supernovae remnant can give us different information about it.
Let's examine the Crab Nebula; it is unique in that it contains one of only a few pulsars that are observable at so many different energies.
The Crab Nebula's creation was witnessed in July of 1054 A.D. when Chinese astronomers and members of the Native American Anasazi tribe separately recorded the appearance of a new star. Although it was visible for only a few months, it was bright enough to be seen even during the day! In the 19th century, French comet hunter Charles Messier recorded a fuzzy ball of light near the constellation Taurus. This fuzzy ball turned out not to be a comet after all, but the remains of a massive star whose explosive death had been witnessed centuries before by the Chinese and the Anasazi.

Scientists now believe the Crab Nebula is the remains of a star which suffered a supernova explosion. The core of the star collapsed and formed a rapidly rotating, magnetic neutron star, releasing energy sufficient to blast the surface layers of the star into space with the strength of a 1028 megaton bomb or a hundred million nuclear warheads. Nestled in the nebulous cloud of expelled gases, the rotating neutron star, or pulsar, continues to generate strobe-like pulses that can be observed at radio, optical, and X-ray energies. The Crab Nebula was one of the first sources of X-rays identified in the early 1960s when the first X-ray astronomy observations were made.
|
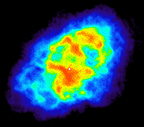
At radio wavelengths, the Crab Nebula, seen to the left, displays two distinctive physical features. The nebulous regions hide radio emission coming from unbound electrons spiraling around inside the nebula. The pulsar at the heart of the Crab Nebula generates pulses at radio frequencies roughly 60 times a second. In this image, the pulsar's flashes are blurred together (since the image was "exposed" for much longer than 1/60 s) and it appears as the bright white spot near the middle of the nebula. |
| In the optical, both a web of filaments at the outer edges of the nebula and a bluish core become apparent. The blue core is from electrons within the nebula being deflected and accelerated by the magnetic field of the central neutron star. The filaments surrounding the edges of the nebula are the remnants of the original outer layers of the star. |

|
|
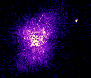
In the ultraviolet (or UV) the nebula is slightly larger than when seen in X-rays. Cooler electrons (responsible for the UV emission) extend out beyond the hot electrons near the central pulsar. This supports the theory that the central pulsar is responsible for energizing the electrons. |
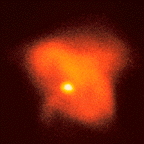
| X-ray observations reveal a condensed core near the central pulsar, which is the bright dot visible slightly left and below center in the image to the right. The Crab Nebula appears smaller and more condensed in X-rays because the electrons which are primarily responsible for the X-ray emission exist only near the central pulsar. Scientists believe that the strong magnetic field near the surface of the neutron star "heats up" the electrons in it and that these "hot" electrons are responsible for the X-ray emission. |
|
| Using the text and any external references, define the following terms: radio waves, microwaves, infrared, visible, ultraviolet, X-rays, gamma rays, light energy, photon, electromagnetic spectrum, electromagnetic radiation, Hertz, wave peak, frequency, and wavelength. |
The EM Spectrum
http://imagine.gsfc.nasa.gov/docs/introduction/emspectrum.html
Back to the Main Spectra Unit Menu