All About the Microcalorimeter
Perhaps the most intriguing advance in X-ray astronomy
instrumentation in the 1990s has been the development of the microcalorimeter,
spearheaded by work at NASA's Goddard Space
Flight Center. The microcalorimeter instrument designed at Goddard was called
XRS, short for the X-ray Spectrometer.
What is a Microcalorimeter?
|
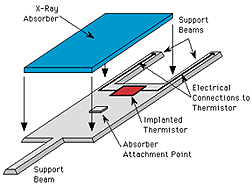
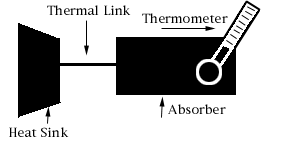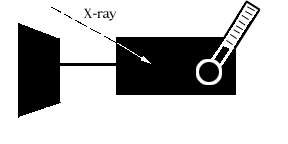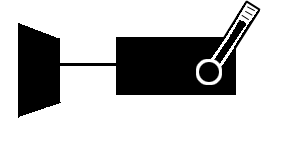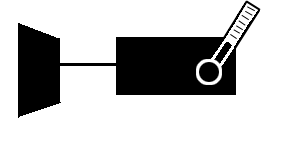
A microcalorimeter is basically a thermal device made of an
absorber, a thermistor, and a heat sink.
The absorber must do 3 things:
absorb X-rays from space efficiently, quickly, completely convert
the absorbed energy into heat (thermalize the energy), and
have a low heat capacity.
There is no material known which excels at all 3 of these properties,
so choosing the absorber material involves deciding on the best
combination of them.
A thermistor is a
device that changes its electrical resistance dramatically
with a small change in temperature. Since a thermometer is any device
that measures temperature, a thermistor is a kind of
thermometer.
|
The combination of the absorber and the thermistor is what we call the
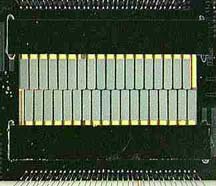
"X-ray detector". Below, starting from the left, is a diagram of a detector,
a photo of a detector array (they are the gray-green rectangles in the middle),
and a closeup of the array. You can (barely) see two black legs from each
detector at the top of the image, and one leg from each detector squeezing
between a pair of detectors at the bottom. These legs of the detector
are what is called the "weak link" to the heat sink.
|
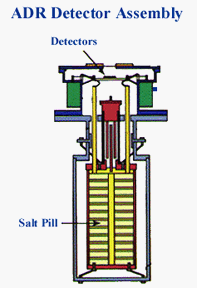
The heat sink is what absorbs heat from the detector, keeping it cool.
In the case of a recently designed XRS, the heat sink used to keep the
detector cool
enough to work was a refridgeration unit called the Adiabatic
Demagnetization Refrigerator (ADR). Inside the ADR was a special "pill" of
salt that did the actual heat absorption. |
 |
How Does a Microcalorimeter Work?
|
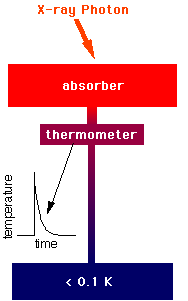
An X-ray photon hits the absorber and
knocks an electron loose from an atom of the
absorber material. This
photoelectron (so-called because a photon of light knocked it loose)
rattles around in the absorber,
ultimately raising the temperature of the absorber by a few milliKelvin (that
is, a few thousandths of one degree Kelvin). The
temperature-sensitive thermistor is partially isolated from the absorber,
to give the absorber time to come into equilibrium before the
thermistor begins to see the temperature rise. After a few milliseconds,
the thermistor comes to the same temperature as the absorber, a few
milliKelvin warmer than the heat sink, which is at 65 milliKelvin.
We know it's
a little strange to be talking about 'heat' when something is near
absolute zero! Next, the thermistor begins to
cool as the heat flows out the weak link (the "legs" of the detector)
to the heat sink. After a few tens of milliseconds, the thermistor has
returned to its normal operating temperature.
|
|
The temperature rise (delta T) measured by the
thermistor is approximately proportional to the energy of the X-ray
photon:
delta T ~ E/C
where delta T is the change in temperature,
E is the energy of the X-ray and C
is the heat capacity of the absorber. So by measuring how much the
temperature changes, we can determine the energy of the X-ray.
The Science
When an X-ray stops in a detector, it gives all of its energy
to one electron. That electron can rattle around in the detector and
give energy to other electrons. All these excited electrons
would rather go back to their original energy. They want to return
to what is called the 'ground state'. Through scattering with other
electrons or with vibrations in the solid itself, they can lose that
extra energy. But that energy has to go somewhere. What it does
is heat the solid and increase its temperature. If you measure the change
in temperature, you can measure how much energy the X-ray originally
had.
How are heat and energy and temperature all related? Heat is a
manifestation of energy. Heat and energy are measured in the same
units (Joules). We usually think of energy as heat if we are thinking
of the total energy of a large ensemble of objects that can exchange
energy with each other and come into equilibrium. So, when an
X-ray photon heats a solid, it gives its energy to the whole solid.
On average, each atom is vibrating a little bit more than before the
X-ray hit. Temperature is the way we relate the total energy of
a system to its state of disorder (entropy). A physical property
called "heat capacity" tells us how much the temperature rises in
a material if we put in a certain amount of energy.
Suppose we wanted to measure the temperature increase due to an
X-ray photon being absorbed. We'd want a very sensitive thermometer,
something that had some physical property that changed a lot for
a small change in temperature. And we'd want the detector to have
a small heat capacity, so its temperature would change a lot for
a small change in energy. And you'd want to do the whole thing
at very low temperatures, because at room temperature there would
already be too much thermal energy in your detector to see the
very small change in energy from the X-ray. That is what an X-ray
calorimeter does. It uses a silicon thermistor which has an electrical
resistance which changes dramatically with small changes in temperature.
It has a low heat capacity. And it operates at less than 0.1 K.
That's Kelvin. Zero on the Kelvin scale is an absolute zero and
represents the cessation of all thermal vibrations. Water freezes
at 273 K. Nitrogen liquifies at 77 K. Helium liquifies at 4 K.
and we operate calorimeters at less than 0.1 K! Calorimeters
are able to get the best spectral resolution of any non-dispersive
spectrometer.
Why is the microcalorimeter a better way to detect X-ray photons?
In other types of detectors (like proportional counters and CCDs),
the energy of the X-ray phton is shared among many electrons.
Each 'excited' electron carries a typical amount of energy, which is
3.65 eV in the case of silicon-based
CCDs. So, an incident X-ray photon with an energy of 3.65 keV will
produce 1,000 electrons --- give or take. There is an uncertainty
here, because the details of the X-ray - matter interaction is different
each time, giving a slightly different amount of energy to each excited
electron. The uncertainty is about the square root of the average number
of electrons --- 30 or so in this case. Since the detector reads out
the number of excited electrons, this sets the theoretical limit on
how accurately you can measure the energy of the X-ray photons (about
3% for 3.65 keV X-rays in a CCD). There doesn't seem to be a detector
material in which an electron's share of energy is much smaller than
in the CCDs. Microcalorimeters, by relying on a completely different
principle, circumvents this limitation and achive much higher spectral
resolution.
| Using the text, and any external printed references,
define the following terms: Kelvin, thermistor, heat capacity.
|
Reference URLs:
Microcalorimeters
http://imagine.gsfc.nasa.gov/docs/science/how_l2/calorimeters.html
http://imagine.gsfc.nasa.gov/docs/science/how_l2/calorimeters.html
http://lheawww.gsfc.nasa.gov/docs/xray/astroe/ae/calorim-details.htm
Thermodynamics
http://www.unidata.ucar.edu/staff/blynds/tmp.html
Back to the Main Spectra Unit Menu