Introduction to Spectroscopy
| Spectroscopy is a complex art - but it can be very useful in helping scientists
understand how an object like a black hole, neutron star, or active galaxy
is producing light, how fast it is moving, and even what
elements it is made of. A spectrum is simply a chart or a graph that
shows the intensity of light being emitted over a range of eneriges. Spectra
can be produced for any energy of light - from low-energy radio waves to
very high-energy gamma-rays.
|
Spectra are complex because each spectrum holds a wide variety of
information. For instance, there are many different mechanisms by which an
object, like a star, can produce light - or using the technical term for
light, electromagnetic radiation. Each of these mechanisms has a
characteristic spectrum.
|
Let's look at a spectrum and examine each part of it.
To the right is an X-ray spectrum made using data from the ASCA satellite.
It is of a supernova remnant (SNR) - a SNR is a huge cloud of gaseous
matter swept up from the explosion of a massive star. The X-axis shows
the range of energy of light that is being emitted. The Y-axis of the graph
shows the intensity of the light recorded by the instrument from the SNR -
- that is, the number of photons of light the SNR is giving off at each energy,
multiplied by the sensitivity of the instrument at that energy.
We can tell that the light, or radiation, from this SNR is very high energy -
if we look at the units of the X-axis - we can see that the photons of light
have energys measured in keV, or kilo-electron Volts.
A kilo-electron Volt
is 1000 electron Volts (eV). This puts is the X-ray range of the
electromagnetic spectrum.
|
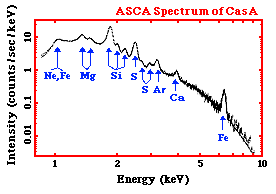 |
The graph shows a decreasing curve, with lots of bumps in it. The
curve itself is called a continuum - it represents X-ray photons
emitted at all energies continuously.
The X-rays that are producing this continuum can be caused by several
mechanism that are completely different than those producing the X-rays
at the
various peaks and bumps on the curve. The peaks and bumps are called line
emission. Not only are these two different kind of X-ray emission
(continuum and line) produced differently, but they each tell us
different things about the source that is emitting them.
The Electromagnetic Spectrum
White light (what we call visible or optical light) can be split up
into its constituent colors easily and with a familiar result - the
rainbow. All we have to do is use a slit to focus a narrow beam of
the light at a prism. This set-up is actually a basic spectrometer.

The resultant rainbow is really a continous spectrum that shows us the
different energies light (from red to blue) present in
visible light. But the electromagnetic spectrum encompasses more
than just
optical light - it covers all energies of light extending from
low-energy radio waves, to microwaves, to infrared, to optical light,
to ultraviolet, to very high-energy X- and gamma-rays.
Line Emission
Instead of using our spectrometer on a light bulb, what if we were to use it to
look a tube of gas - for example, hydrogen? We would first need to
heat the hydrogen to very high temperatures, or give the atoms of hydrogen
energy by running an electric current through the tube. This would cause
the gas to glow - to emit radiation. If we looked at the spectrum of light
given off by the hydrogen gas with our spectroscope, instead of seeing a
continuum of colors, we would just see a few bright lines. Below we see
the spectrum, the unique fingerprint of hydrogen.

These bright lines are called emission lines. Remember how we heated
the hydrogen to give the atoms energy? By doing that, we excited the electrons
in the atom - when the electrons fell back to their ground state, they gave
of photons of light at hydrogen's
characteristic energies. If we altered the
amount or abundance of hydrogen gas we have, we could change the
intensity of the lines, that is, their brightness, because more photons would
be produced. But we couldn't
change their color - no matter how much or how little hydrogen gas was
present, the pattern of lines would be the same. Hydrogen's pattern
of emission lines is unique to it. The brightness of the emission
lines can give us a great deal of information about the abundance of hydrogen
present. This is particularly useful in a star, where
there are many elements mixed together.
Each element in the periodic table can appear in gaseous form and will each
produce a series of bright emission lines unique to that element. The
spectrum of hydrogen will not look like the spectrum of helium, or the
spectrum of carbon, or of any other element.
Hydrogen:

Helium:

Carbon:

We know that the continuum of the electromagnetic spectrum extends from
low-energy radio waves, to microwaves, to infrared, to optical light,
to ultraviolet, to X and gamma-rays. In the same way, hydrogen's unique
spectrum extends over a range, as do the spectra of the other elements.
The above spectra are in the optical range of light.
Line emission can actually occur at any energy of light (i.e. visible, UV, etc.
) and with any type of atom, however, not all atoms have line
emission at all wavelengths. The difference in energy between levels
in the atom is not great enough for the emission to be X-rays in
atoms of lighter elements, for example.
Different Graphical Representations of Spectra
The sample spectra above represent energy emission as lines, the amount of
photons of light represented by the brightness and width of the line.
But we can also
make a graphical representation of a spectrum. Instead of the emission of
a characteristic energy being shown as a line, it can be shown as a peak
on a graph. In this case, the height and width of the peak show its
intensity. One example of this is the very first spectrum we looked at - the
one of the supernova remnant. The peaks and bumps on the graph are simply
a graphical representation of the emission lines of different elements.
Below, you will see the spectrum of the Sun
at ultraviolet wavelengths. There are distinct lines (in the top
graph) and peaks (in the bottom one) and if you look at the X-axis,
you can see what energies they correspond to. For example, we know
that helium emits light at a wavelength of 304 Angstroms, so if we see
a peak at that wavelength, we know that there is helium present.
Spectra and Astronomy
In a star, there are actually many elements present. The way we can tell
which ones are there is by looking at the spectra of the star. In fact, the
element helium was first discovered in the Sun, before it was ever
discovered on Earth. The element is named after the Greek name for the Sun,
Helios.
The science of spectroscopy is quite sophisticated. From spectral
lines astronomers can determine not only the element, but the
temperature and density of that element in the star. Emission lines can
also tell us about the magnetic field of the star. The width of
the line can tell us how fast the material is moving, giving us
information about stellar wind. If the lines shift back and forth, it means
that the star may be orbiting another star - the spectrum will give
the information necessary to estimating the mass and size of the star system
and the companion star. If the lines grow and fade in
strength we can learn about the physical changes in the star.
Spectral information, particularly from energies of light other than
optical, can tell us about material around stars. This material may
have been pulled from a companion star by a black hole or a neutron
star, where it will form an orbiting disk. Around a compact object
(black hole, neutron star), the material in this
accretion disk is heated to the point that it gives off X-rays,
and the material eventually falls onto the black hole or neutron
star. It is by looking at the spectrum of X-rays being emitted by
that object and its surrounding disk, that we can learn about the nature
of these objects.
Continuum Emission

Just like visible light, with its range of energies from red to blue,
X-rays have a continuum, or a range of energies associated with it.
X-rays usually range in energy from around 0.5 keV up to around 1000 keV.
Like line emission, continuum X-ray emission involves charged particles.
Continuum emission is a result of the acceleration of a population of
charged particles.
All X-ray sources contain such particles. These particles must be
at least partially ionized - their electrons need to be unbound from their
nuclei to be free to zip around when they are heated to
extreme temperatures. For an electron
to radiate X-rays, the gas containing the electron
must have extreme conditions, such as temperatures of millions of degrees,
super-strong magnetic fields, or the electrons themselves must be moving
at nearly the speed of light. Extreme conditions
can be found in disks of matter orbiting black holes or in supernova remnants.
Strong magnetic fields, like those created in the wake of a supernova
explosion, can also accelerate fast moving ions in spirals around the
field lines to the point of X-ray emission. Electrons can be accelerated
to nearly the speed of light in the shockwave created by a supernova explosion.
There are three mechanisms that will produce continuum X-ray emission.
They are Synchrotron Radiation, Bremsstrahlung, and Compton
Scattering. Because the populations of electrons have a continuous
range of energies, and they can be accelerated through a range of energies,
the radiation produced is continuous, and not at the discreet energies of
line emission.
courtesy of University of Hertfordshire |
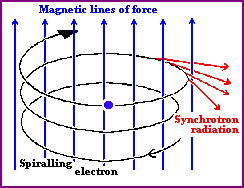
Sychrotron radiation is emitted when a fast electron
interacts with a magnetic field. A magnetic field in an area an
electron is traveling in will cause the electron to change direction by
exerting a force on it perpendicular to the direction the electron is moving.
As a result, the electron will be accelerated, causing it to radiate
electromagnetic energy. This is called magnetic
bremsstrahlung or synchrotron radiation (after radiation
observed from particle accelerators by that name). If the electrons and the
magnetic field are energetic enough, the emitted radiation can be in the form
of X-rays.
|
|
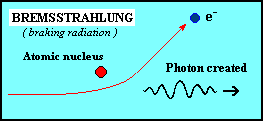
Bremsstrahlung occurs when an electron passes close to a
positive ion, and the strong electric forces cause its trajectory to
change. The acceleration of the electron in this way causes it to
radiate electromagnetic energy - this radiation is called
bremsstrahlung, (literally, from the German meaning 'braking
radiation'). Thermal bremsstrahlung occurs in a hot gas, where many
electrons are stripped from their nuclei, leaving a population of
electrons and positive ions. If the gas is hot enough (millions of
degrees Kelvin), this kind of radiation will primarily take the form
of X-rays. |
courtesy of University of
Hertfordshire |

courtesy of University of Hertfordshire |
|
Comptonization is when a photon collides with an electron - the photon
will either give up energy to or gain energy from the electron,
changing the electron's velocity as a result.
|
What Are Some Examples of This In Action?
Gas that is hotter than 10 million degrees, such as the gas heated by a supernova
explosion, produces most of its emission in X-rays from thermal Bremsstrahlung.
Gas can be heated to these temperatures by the outward moving shock of a supernova
explosion, or in an accretion disk around a black hole or neutron star. Synchrotron
radiation can produce X-rays around supernova remnants (SNR), where the magnetic
fields are strong and ions have been accelerated by the shock wave to high energies.
X-rays produced by SNR require electrons with energies of about 104
GeV each (you would have to heat an electron to a temperature of about ten trillion
degrees for it to have this much energy)! Synchrotron radiation and Compton
scattered radiation are major components of the diffuse X-ray background and
emission from active galaxies.
| Using the text, define the following terms:
spectroscopy, keV, continuum, continuum emission, line emission,
electromagnetic spectrum, synchrotron radiation, bremmstrahlung,
comptonization.
|
Reference URLs:
Spectroscopy
http://imagine.gsfc.nasa.gov/docs/science/how_l1/spectra.html
http://www.colorado.edu/physics/PhysicsInitiative/Physics2000/quantumzone/
Back to the Main Spectra Unit Menu







