Graphing Spectra - A Student Worksheet
Part II.
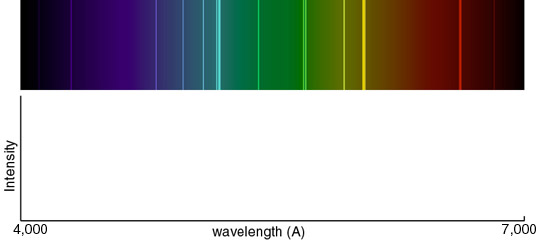
The following
spectrum represents the energy state of the element, carbon. Carbon's
emission lines in the visible range are a function of wavelength from
4,000
to 7,000 Angstroms. You are going to create a graphical representation of
carbon's spectrum from the photographic representation. Refer to the
example above to help. At the particular wavelengths, illustrate the
varying brightness of carbon's emission lines. Notice that in the
photographic representation of the spectrum there is an underlying
continuum of emission, in addition to the bright spectral lines. This
continuum is due to contamination of the spectrum by ambient light, such
as small amounts of white light that are picked up by the spectrometer.
Your graphical representation should include this low level of emission at
all wavelengths as well as carbon's spectral line features.
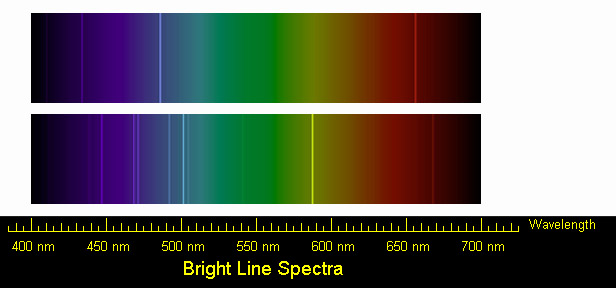
Below you are given spectra for both hydrogen and helium. For each
element, select two of the brightest emission lines at the particular
wavelengths and measure the wavelengths. The scale of the spectrum is
indicated by the ruler below. Solve for the frequency and energy of these
lines, using the
relationships between wavelength and requency and between frequency and
energy. (Hint: You will have to manipulate an equation.) After the flame
test, you will complete the same calculations for the following elements:
sodium and calcium.