Procedure:
Cover the ball-bearing or bead with one color of clay - make the layer of clay
at least half an inch thick. Use another color of clay to make a layer
over the first. Do this until you have 8 layers of clay, each a different
color, each at least half an inch thick. Now, cut the ball in half to
make a cross section (you'll have to cut around the ball-bearing). The
inside shows the different layers present in the core of a high-mass star.
Each of those layers of clay
belongs to a different element. The ball-bearing is the iron core of the
star. At the end of the day's activity, the class will come back to the model
and learn what the different layers are.
Exploration
Materials:
Index cards with elements written on them. You'll need
4 hydrogen-1 (1H)
13 helium-4 (4He)
4 carbon-12 (12C)
1 magnesium-24 (24Mg)
4 oxygen-16 (16O)
1 sulphur-32 ( 32S)
1 neon-20 (20Ne)
1 silicon-28 (28Si)
2 nickel-56 (56Ni)
2 cobalt-56 (56Co)
2 iron-56 (56Fe)
2 iron-57 (57Fe)
2 iron-58 (58Fe)
1 iron-59 (59Fe)
3 neutrons (n)
4 positrons (e+)
2 neutrinos
at least 7 energy
Give each student an index card with an element written on it. Have the students move about the classroom and construct fusion reactions. Their goal is to form the reactions that create helium, carbon, magnesium, oxygen, sulphur, neon, nickel, cobalt, and 4 different isotopes of iron. The teacher should assist or give hints as necessary.
The students should end up with the following fusion relationships:
|
4 (1H) ------> 4 He + 2 e+ +
2 neutinos + energy 3 (4He) ------> 12C + energy 12C + 12C ------> 24Mg + energy 12C + 4 He ------> 16O + energy 16O + 16O ------> 32S + energy 16O + 4 He -----> 20Ne +energy 28Si + 7(4 He) ------> 56Ni + energy 56Ni ------> 56Co + e+ (postive Beta Decay) 56Co ------> 56Fe + e+ (positive Beta Decay) 56Fe + n ------> 57Fe 57Fe + n ------> 58Fe 58Fe + n ------> 59Fe
|
When the students create a correct reaction, write it on the board - keep the reactions in the order they are in the table above. The order is important.
|
Adapting for class size: The number of cards handed out will vary depending on class size. If your class size is small, only do 2 or 3 reactions at a time, handing out only the cards with elements that are in those reactions. Just be sure every student has a card. If your class size is large, do about half the reactions at a time, giving the students only the cards used in those reactions. If there are not enough cards to go around, give out extra energy cards. It is alright to have more than one student representing energy in a reaction. When the students are done, collect those cards and hand out the cards used in the rest of the fusion reactions and have the class form them. |
When the class is done forming reactions, have them examine the reactions and their order. They should see, that like high-mass stars, they have created heavy elements, even though they started with just hydrogen. A high-mass star converts its hydrogen to helium, helium to carbon, carbon to magnesium, carbon and helium to oxygen, oxygen to sulphur, oxygen and helium to neon, and silicon and helium to nickel. The unstable isotope of nickel created undergoes postive beta decay and forms an isotope of cobalt that in turn decays into iron. Positive beta decay is when a proton becomes a neutron, and a positron is emitted. A high-mass star creates many unstable isotopes of iron and actually goes through a series of reactions that cause the star to make heavier and heavier nuclei of elements, all the way up to bismuth-209 - the heavest known nonradioactive nucleus.
This process is the origin of the copper and silver in the coins in our pockets, the lead in our car batteries, and the gold in the rings on our fingers!
Now that the class is aware of the order in which the elements are created in a star, bring them back to the model of the core of the star from the beginning of class. The ball-bearing is the iron core of the star. The layers outside it are where various nuclei fuse. Have the students associate a layer of clay with an element that is being produced by the high-mass star. This will illustrate that as the temperature of the star increases with depth, the ash of each burning stage becomes the fuel for the next stage. Surrounding the core of iron nuclei is a later of silicon fusion, then magnsium, then neon, then oxygen, then carbon, then helium, and lastly, in the relatively cool periphery of the core, hydrogen fuses into helium. The core is enveloped by a layer of nonburning hydrogen.
Have the class draw their own version of the onion-like nature of the core of a star based on the model and explain the process that occurs at each layer for homework. Here is an example:
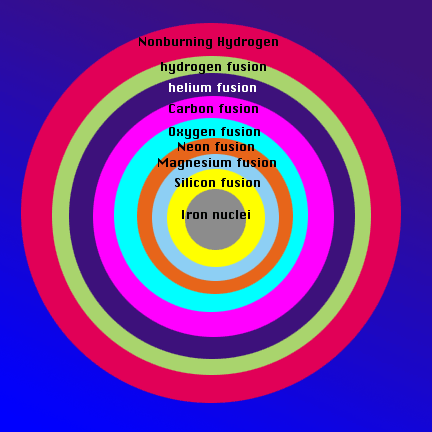
Evaluation
Have each group of students explain the reaction they have made and why they think it is correct. Their individual diagrams and explanations of the core of a high-mass star may also be evaluated.
Reference URLs:
Element Production
http://zebu.uoregon.edu/disted/ph123/l10.html
http://aether.lbl.gov/www/tour/elements/element.html
http://astro.uchicago.edu/home/web/mohr/Compton/HTML_three/sld011.html
http://zebu.uoregon.edu/~soper/Sun/fusion.html
http://zebu.uoregon.edu/~soper/Sun/fusionsteps.html
Reference Book:
Astronomy Today, by Eric Chaisson and Steve McMillan.
Back to the Main Spectra Unit Menu