Answer This!
|
| Click here for a list of constants |
| Wavelength (m) | Frequency (Hz) | Energy (J) | Energy (KeV) | Electromagnetic Radiation Range |
E = hn
energy = Planck's constant x frequency
Answer This!
|
| Click here for a list of constants |
| Wavelength (m) | Frequency (Hz) | Energy (J) | Energy (KeV) | Electromagnetic Radiation Range |
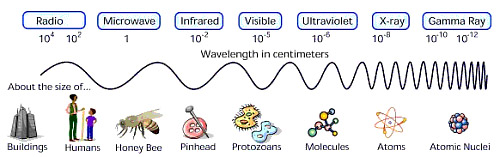
Thought QuestionsIn three minutes, summarize what you have learned about light and the relationship between its energy, frequency and wavelength. Write an unanswered question you still have. |